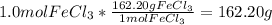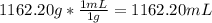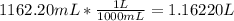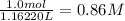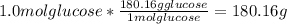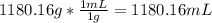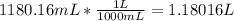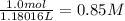Chemistry, 28.06.2019 20:30 hsjsjsjdjjd
If you make a solution by dissolving 1.0 mol of fecl3 into 1.0 kg of water, how would the osmotic pressure of this solution compare with the osmotic pressure of a solution that is made from 1.0 mol of glucose in 1.0 kg of water? one-half as large the same twice as large four times as large

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

You know the right answer?
If you make a solution by dissolving 1.0 mol of fecl3 into 1.0 kg of water, how would the osmotic pr...
Questions

Mathematics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00

History, 19.02.2021 14:00

German, 19.02.2021 14:00


Biology, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00



Mathematics, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00

History, 19.02.2021 14:00


English, 19.02.2021 14:00

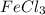 solution:
solution: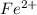 ion and 3
ion and 3 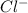 ions.
ions.