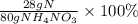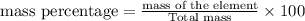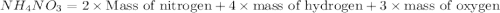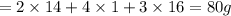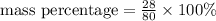Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Which expression represents the percent by mass of nitrogen in nh 4 no 3 ? a. 28 g n / 80 g nh 4 no...
Questions



Computers and Technology, 02.08.2019 22:20





Mathematics, 02.08.2019 22:20


Social Studies, 02.08.2019 22:20




Mathematics, 02.08.2019 22:20


Health, 02.08.2019 22:20

History, 02.08.2019 22:30

Mathematics, 02.08.2019 22:30

Mathematics, 02.08.2019 22:30

English, 02.08.2019 22:30