
Chemistry, 28.06.2019 17:30 Itsyourgirllulu
You wish to measure the iron content of the well water on the new property you are about to buy. you prepare a reference standard fe3 solution with a concentration of 5.17 ă— 10-4 m. you treat 13.0 ml of this reference with hno3 and excess kscn to form a red complex, and dilute the reference to 45.0 ml. the diluted reference is placed in a cell with a 1.00-cm light path. you then take 30.0 ml of the well water, treat with hno3 and excess kscn, and dilute to 100.0 ml. this diluted sample is placed in a variable pathlength cell. the absorbance of the reference and the sample solutions match when the pathlength is 4.78 cm. what is the concentration of iron in the well water? for each solution, the zero is set with a blank.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
You know the right answer?
You wish to measure the iron content of the well water on the new property you are about to buy. you...
Questions




English, 11.07.2019 05:00

Chemistry, 11.07.2019 05:00




Chemistry, 11.07.2019 05:00


Mathematics, 11.07.2019 05:00

Mathematics, 11.07.2019 05:00

History, 11.07.2019 05:00

Arts, 11.07.2019 05:00

Mathematics, 11.07.2019 05:00

Physics, 11.07.2019 05:00

Health, 11.07.2019 05:00

Biology, 11.07.2019 05:00


History, 11.07.2019 05:00

 M
M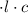 by the Beer-Lambert law, where
by the Beer-Lambert law, where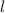 the path length, ε the molar absorptivity of the solute, and
the path length, ε the molar absorptivity of the solute, and concentration of the solution.
concentration of the solution.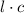 is constant;
is constant; 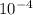 M. Diluting it to 45.0 mL results in a concentration of
M. Diluting it to 45.0 mL results in a concentration of 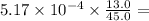 1.494 M.
1.494 M.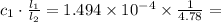 3.126 M.
3.126 M.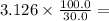 1.04 ⨯
1.04 ⨯ 

