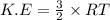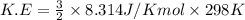Chemistry, 28.06.2019 14:30 dsdlskds3880
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature (25.0ºc).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature...
Questions


SAT, 24.10.2021 02:20


English, 24.10.2021 02:20

Computers and Technology, 24.10.2021 02:20


History, 24.10.2021 02:20


Business, 24.10.2021 02:20

Mathematics, 24.10.2021 02:20


History, 24.10.2021 02:20

Mathematics, 24.10.2021 02:20

English, 24.10.2021 02:20


History, 24.10.2021 02:20

English, 24.10.2021 02:20


Physics, 24.10.2021 02:20