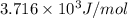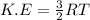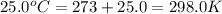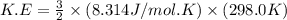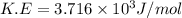Chemistry, 28.06.2019 14:30 ConnorRecck3140
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature (25.0ºc).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature...
Questions



Geography, 19.10.2019 01:00




English, 19.10.2019 01:00




Mathematics, 19.10.2019 01:00

Computers and Technology, 19.10.2019 01:00



Mathematics, 19.10.2019 01:00