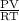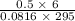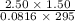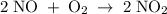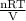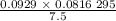Chemistry, 28.06.2019 07:30 nickeymcorrea
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.500 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 °c. what are the partial gasses of no, and no2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l,...
Questions






Arts, 25.07.2019 08:30


English, 25.07.2019 08:30

History, 25.07.2019 08:30


English, 25.07.2019 08:30

Chemistry, 25.07.2019 08:30

Biology, 25.07.2019 08:30

Mathematics, 25.07.2019 08:30

Mathematics, 25.07.2019 08:30


Mathematics, 25.07.2019 08:30


Physics, 25.07.2019 08:30

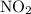 is 0.400 atm at the end of the reaction.
is 0.400 atm at the end of the reaction.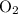 initially are;
initially are;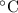 = 273 + 22 K = 295 K
= 273 + 22 K = 295 K