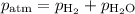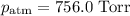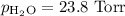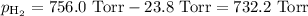Chemistry, 28.06.2019 07:30 emmaty7845
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr and the temperature is 25 °c. the vapor pressure of water at various temperatures can be found in this table. calculate the molar mass of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1


Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291...
Questions


Mathematics, 14.08.2020 08:01

Mathematics, 14.08.2020 08:01

Advanced Placement (AP), 14.08.2020 08:01




Chemistry, 14.08.2020 08:01

History, 14.08.2020 08:01


Mathematics, 14.08.2020 08:01

History, 14.08.2020 08:01


Chemistry, 14.08.2020 08:01


History, 14.08.2020 08:01


Mathematics, 14.08.2020 08:01