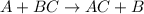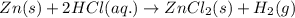Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because zinc is more reactive than hydrogen. a single replacement reaction takes place because zinc is more reactive than chlorine. a double replacement reaction takes place because zinc is less reactive than hydrogen. a double replacement reaction takes place because zinc is less reactive than chlorine.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this rea...
Questions



Mathematics, 29.12.2020 16:00

Mathematics, 29.12.2020 16:00

English, 29.12.2020 16:00


Arts, 29.12.2020 16:00

Mathematics, 29.12.2020 16:00

Social Studies, 29.12.2020 16:10

English, 29.12.2020 16:10

Biology, 29.12.2020 16:10

Social Studies, 29.12.2020 16:10


Social Studies, 29.12.2020 16:10

Social Studies, 29.12.2020 16:10



Mathematics, 29.12.2020 16:10

English, 29.12.2020 16:10

Physics, 29.12.2020 16:10