
Chemistry, 28.06.2019 06:00 jagmeetcheema
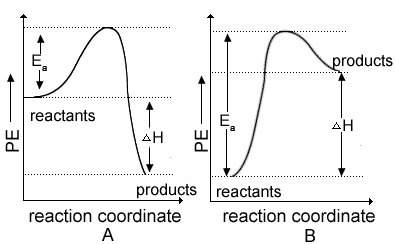
This is due todaystudy the graph below to answer the question. reaction a is an exothermic process and reaction b is an endothermic process because a. the enthalpy change of reaction a is lower than that of reaction b. b. δh is positive for reaction a and δh is negative for reaction b. c. the energy of activation of reaction a is lower than that of reaction b. d. δh is negative for reaction a and δh is positive for reaction b.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 21:30
Hno3 + s → h2so4 + no break down the equation shown into the skeletal half-reactions for oxidation and reduction. which of these pairs shows the two skeletal half-reactions with their correct assignments?
Answers: 3
You know the right answer?
This is due todaystudy the graph below to answer the question. reaction a is an exothermic process a...
Questions



Mathematics, 22.04.2021 22:40


Arts, 22.04.2021 22:40



Mathematics, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40

Biology, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40


Geography, 22.04.2021 22:40






Computers and Technology, 22.04.2021 22:40



