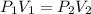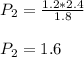Chemistry, 28.06.2019 03:30 vivianni0727p1y30v
Asample of gas has a volume of 2.4 l and a pressure of 1.2 atm. what would the pressure of the same gas sample be if the volume is reduced to 1.8 l at a constant temperature? 0.90 atm 1.6 atm 5.2 atm 3.6 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Asample of gas has a volume of 2.4 l and a pressure of 1.2 atm. what would the pressure of the same...
Questions










Mathematics, 04.04.2020 02:23





Mathematics, 04.04.2020 02:23


Chemistry, 04.04.2020 02:23