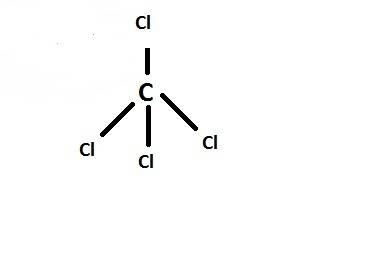
Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a refrigerant. construct an explanation of how carbon and chlorine combine to form carbon tetrachloride. a) nonmetal carbon shares valence electrons with each nonmetal chlorine forming four covalent bonds. b) nonmetal carbon loses a valence electron and chlorine metal gains a valence electron to form an ionic bond. c) carbon and chlorine are nonmetals and they shares their valence electrons to become ions and form ionic bonds. d) chlorine metal loses a valence electron to become a cation and nonmetal carbon gains a valence electron to become an anion forming a covalent bond.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a...
Questions


Mathematics, 15.04.2021 04:40

History, 15.04.2021 04:40


Mathematics, 15.04.2021 04:40


Chemistry, 15.04.2021 04:40


English, 15.04.2021 04:50

Mathematics, 15.04.2021 04:50


Chemistry, 15.04.2021 04:50


Mathematics, 15.04.2021 04:50



Chemistry, 15.04.2021 04:50

Mathematics, 15.04.2021 04:50

Physics, 15.04.2021 04:50

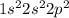 . Carbon has 4 valence electrons. It can only share electrons as it is difficult to gain or lose 4 electrons to complete it's octet.
. Carbon has 4 valence electrons. It can only share electrons as it is difficult to gain or lose 4 electrons to complete it's octet. 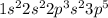 . It has 7 valence electrons and need one electron to complete its octet.
. It has 7 valence electrons and need one electron to complete its octet.