
Chemistry, 28.06.2019 03:00 jetblackcap
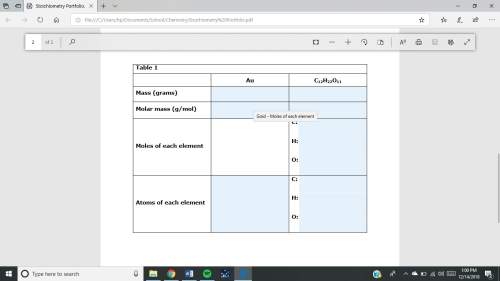
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the sample and record in table 1. show your work. b. calculate the number of atoms of gold (au) in the sample and record in table 1. show your work. 2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample and record in table 1. show your work. b. calculate the moles of each element in c12h22o11 and record in table 1. show your work. c. calculate the number of atoms of each type in c12h22o11 and record in table 1. show your work. table looks like this: column 1 column 2 column 3 au c12h22o11mass (grams)molar mass (g/mol)moles of each element c: h: o: atoms of each element c: h: o: fast!

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1


Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the...
Questions


History, 11.10.2019 00:00

English, 11.10.2019 00:00

Mathematics, 11.10.2019 00:00

English, 11.10.2019 00:00


Mathematics, 11.10.2019 00:00

Mathematics, 11.10.2019 00:00


Mathematics, 11.10.2019 00:00







Mathematics, 11.10.2019 00:00

Geography, 11.10.2019 00:10


History, 11.10.2019 00:10



