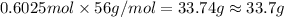And explain how to get the answer! 1. given the equation: 2na+cl2--> 2nacl if 200 grams of naci is produced, how many grams of na must be reacted with excess chlorine? a. 58.43g nab. 78.65g nac. 22.98g nad. 3.4g na2. given the equation: 2k+2h2o--> 2koh+h2 if 23.5 grams of potassium are reacted with excess water, how many grams of potassium hydroxide will be formed? a. 33.7g kohb. 56.08g kohc. 39.09g kohd. 17.99g koh

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
And explain how to get the answer! 1. given the equation: 2na+cl2--> 2nacl if 200 grams of naci...
Questions


Mathematics, 01.07.2021 15:40


History, 01.07.2021 15:40








English, 01.07.2021 15:40





Social Studies, 01.07.2021 15:40



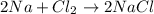
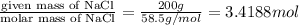
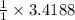 moles of Na.
moles of Na.
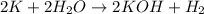
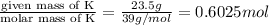
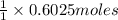 of KOH
of KOH