
Chemistry, 29.09.2019 00:00 chessacs2950
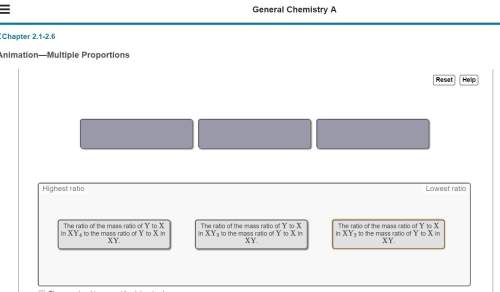
The elements x and y combine in different ratios to form four different types of compounds: xy, xy₂, xy₃, and xy₄. consider that there is enough of each sample to contain 2 g of x, and the mass of x is estimated to be 2 g and the mass of y is estimated to be 4 g.
arrange the following ratios in order of their increasing value. (the exercise gives me three tabs to put in order from highest to least. these are in the pic)
rank from highest to lowest ratio. to rank items as equivalent, overlap them.
i put xy₄ as highest, then xy₃ in the middle, and xy₂ as lowest. is this order correct? i'm not quite understanding this lesson : // so if i could also get some sort of an explanation, that'd be

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
The elements x and y combine in different ratios to form four different types of compounds: xy, xy₂...
Questions


English, 17.03.2021 23:50






Mathematics, 17.03.2021 23:50




Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50


Biology, 17.03.2021 23:50



History, 17.03.2021 23:50



