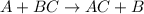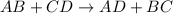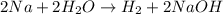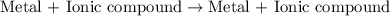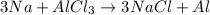Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can replace aluminum (al) in the compound because lead is lower on the activity series. true false question 2(multiple choice worth 4 points) (04.03 mc) which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh? na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced question 3(multiple choice worth 4 points) (04.03 lc) which of the following is a single replacement reaction? ba(oh)2 + h2so4 → baso4 + 2h2o 2mg + o2 → 2mgo h2o+ co2 → h2co3 zn + h2so4 → znso4 + h2 question 4(multiple choice worth 4 points) (04.03 mc) sodium metal reacts with water to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is more reactive than hydrogen. a single replacement reaction takes place because sodium is more reactive than hydrogen. question 5 (true/false worth 2 points) (04.03 lc) a single replacement reaction is a reaction in which one element replaces a similar element within a compound. true false question 6(multiple choice worth 4 points) (04.03 mc) the table shows the nature of reactants and products formed in a certain type of chemical reaction. nature of reactants and products reactants products metal + ionic compound metal + ionic compound which of the following is true about the type of chemical reaction? it is a single replacement reaction, and the anions in the two ionic compounds are different. it is a single replacement reaction, and the cations in the two ionic compounds are different. it is a double replacement reaction, and the anions in the two ionic compounds are different. it is a double replacement reaction, and the cations in the two ionic compounds are different.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can re...
Questions




French, 25.09.2020 08:01

Computers and Technology, 25.09.2020 08:01



Mathematics, 25.09.2020 08:01



Mathematics, 25.09.2020 08:01



Arts, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01


Advanced Placement (AP), 25.09.2020 08:01


Mathematics, 25.09.2020 08:01

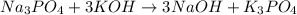 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction.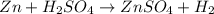
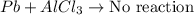
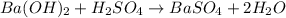 : This equation is a type of double displacement reaction.
: This equation is a type of double displacement reaction.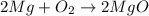 : This equation is a type of combination reaction.
: This equation is a type of combination reaction.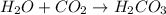 : This equation is a type of combination reaction.
: This equation is a type of combination reaction.