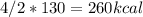Chemistry, 27.06.2019 15:30 hahalol123goaway
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced equation: 2cl2(g) + 7o2(g) + 130kcal -> 2cl2o7(g) a. 1040 kcal b. -260 kcal c. 260 kcal d. -1040 kcal ** if you could explain it as well, that would be much appreciated if not, thats okay too its multiple choice

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced eq...
Questions

Mathematics, 12.02.2021 14:00

Biology, 12.02.2021 14:00

Health, 12.02.2021 14:00











Mathematics, 12.02.2021 14:00

Medicine, 12.02.2021 14:00



Biology, 12.02.2021 14:00

English, 12.02.2021 14:00

Physics, 12.02.2021 14:00

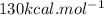
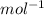 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product 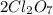 we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.