
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in a large beaker. the solution still looked clear like water, but when the students carefully touched the beaker one at a time, it felt warm to the touch. why did the beaker most likely feel warm? a. the two liquids were not soluble in water. b. the release of a gas heated the solution. c. the energy of mixing warmed the solution. d. a chemical reaction was producing a new substance.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in...
Questions

Mathematics, 18.03.2021 02:40




Biology, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Computers and Technology, 18.03.2021 02:40

Geography, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

History, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40





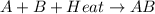
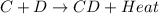 is an exothermic reaction.
is an exothermic reaction.


