
Chemistry, 27.06.2019 10:00 maelaysiap
Water is produced from the reaction of hydrogen and oxygen gas, according to the equation below. what is the excess reactant in the reaction of 4.2 moles of hydrogen with 3.0 moles of oxygen? 2h2(g) + o2(g) → 2h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
Water is produced from the reaction of hydrogen and oxygen gas, according to the equation below. wha...
Questions




Mathematics, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20

History, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20


Mathematics, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20



Mathematics, 03.12.2020 22:20

Social Studies, 03.12.2020 22:20


Mathematics, 03.12.2020 22:20

Social Studies, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20

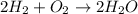
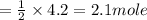 of oxygen gas.
of oxygen gas.

