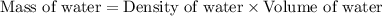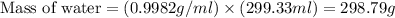Chemistry, 27.06.2019 08:30 quintinjerome
Hurry ! calculating - a sample it barium nitrate is placed into a jar containing water. the mass of the barium nitrate sample is 27g. assume the water is at 20°c and that the resulting barium nitrate solution is saturated. what mass of water is present in the jar?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Hurry ! calculating - a sample it barium nitrate is placed into a jar containing water. the mass of...
Questions



Advanced Placement (AP), 28.07.2019 04:33

History, 28.07.2019 04:33

Physics, 28.07.2019 04:33



Mathematics, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33

History, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33



History, 28.07.2019 04:33





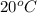 is 9.02 gram per 100 ml of water.
is 9.02 gram per 100 ml of water.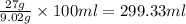 of water
of water