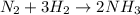Chemistry, 27.06.2019 04:30 anthonybowie99
How do balanced chemical equations show the conservation of mass? they show that the atoms in the products may be different than the reactants as long as the mass does not change. they show that the atoms in the products are the same as in the reactants, but the number of atoms must change. they show that the number of atoms of each element is the same in the products and

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
How do balanced chemical equations show the conservation of mass? they show that the atoms in the p...
Questions

Mathematics, 26.11.2019 08:31

English, 26.11.2019 08:31



Biology, 26.11.2019 08:31

English, 26.11.2019 08:31

Mathematics, 26.11.2019 08:31


Mathematics, 26.11.2019 08:31


Mathematics, 26.11.2019 08:31



Physics, 26.11.2019 08:31


English, 26.11.2019 08:31

Geography, 26.11.2019 08:31

History, 26.11.2019 08:31


English, 26.11.2019 08:31