
Chemistry, 27.06.2019 04:30 saskiat1155
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-], and hydronium, [h3o+], ions. b) pure water contains larger amounts of hydroxide, [oh-], ions than hydronium, [h3o+], ions. c) pure water contains larger amounts of hydronium, [h3o+], ions than hydroxide, [oh-], ions. d) pure water is an electrolyte.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-]...
Questions

History, 02.07.2019 23:10

Mathematics, 02.07.2019 23:10

Mathematics, 02.07.2019 23:10
















Mathematics, 02.07.2019 23:20

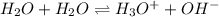
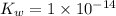
![[H_3O^+]=[OH^-]](/tpl/images/0022/0619/f28a0.png)
![K_w=1\times 10^{-14}=[H_3O^+]\times [OH^-]](/tpl/images/0022/0619/a50f9.png)
![[H_3O^+]=10^{-7}](/tpl/images/0022/0619/e211a.png)
![pH=-\log[H_3O^+]=-\log[10^{-7}] = 7](/tpl/images/0022/0619/ddc2d.png)


