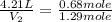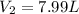Chemistry, 27.06.2019 03:00 paulesparsa6
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the same temperature and pressure?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the s...
Questions

Mathematics, 11.11.2021 01:00




Mathematics, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00

English, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00

Chemistry, 11.11.2021 01:00

Business, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00

Medicine, 11.11.2021 01:00

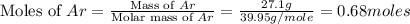
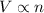
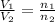
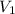 = volume of argon gas
= volume of argon gas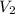 = volume of neon gas
= volume of neon gas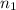 = number of moles of argon gas
= number of moles of argon gas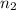 = number of moles of neon gas
= number of moles of neon gas