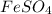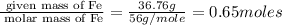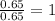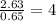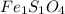Chemistry, 27.06.2019 03:00 ronaldhernandez598
What is the empirical formula? a compound is used to treat iron deficiency in people. it contains 36.76% iron, 21.11% sulfur, and 42.13% oxygen. the empirical formula is feso. reset next

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
What is the empirical formula? a compound is used to treat iron deficiency in people. it contains 3...
Questions


Mathematics, 17.07.2019 03:00



History, 17.07.2019 03:00

English, 17.07.2019 03:00



Mathematics, 17.07.2019 03:00

Mathematics, 17.07.2019 03:00

Mathematics, 17.07.2019 03:00

Mathematics, 17.07.2019 03:00



Mathematics, 17.07.2019 03:00


Biology, 17.07.2019 03:00

Health, 17.07.2019 03:00

Mathematics, 17.07.2019 03:00

Geography, 17.07.2019 03:00