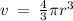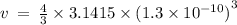Chemistry, 27.06.2019 02:00 msbanks317
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calculate the fraction of space that xe atoms occupy in a sample of xenon at stp.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3



You know the right answer?
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calcula...
Questions



Business, 06.10.2019 03:30


History, 06.10.2019 03:30



Mathematics, 06.10.2019 03:30


History, 06.10.2019 03:30




Mathematics, 06.10.2019 03:30

Mathematics, 06.10.2019 03:30


English, 06.10.2019 03:30