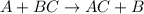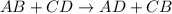Chemistry, 26.06.2019 20:30 johndacres8280
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbonate? it is a single replacement reaction because two new compounds of calcium and sodium are formed. it is a double replacement reaction because calcium chloride and sodium carbonate exchange their non-metallic parts. it is a single replacement reaction because calcium replaces sodium within a compound as sodium is higher than calcium in the activity series. it is a double replacement reaction because the ions of calcium and sodium exchange places in an aqueous solution as calcium is higher than sodium in the activity series.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3


Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

You know the right answer?
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbon...
Questions



Mathematics, 09.06.2021 06:30

Mathematics, 09.06.2021 06:30


Chemistry, 09.06.2021 06:30


Business, 09.06.2021 06:30

Mathematics, 09.06.2021 06:30

English, 09.06.2021 06:30

Engineering, 09.06.2021 06:30


English, 09.06.2021 06:30


Mathematics, 09.06.2021 06:30





Chemistry, 09.06.2021 06:30