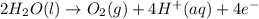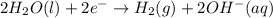Chemistry, 26.06.2019 19:30 michael2737
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the anode through reduction reactions. hydrogen cations give electrons to the anode through oxidation reactions. electrons from the cathode combine with hydrogen cations through reduction reactions. electrons from the cathode combine with hydrogen cations through oxidation reactions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the ano...
Questions

Chemistry, 08.10.2019 05:30


History, 08.10.2019 05:30

Geography, 08.10.2019 05:30

History, 08.10.2019 05:30

History, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30

Chemistry, 08.10.2019 05:30

History, 08.10.2019 05:30




Mathematics, 08.10.2019 05:30





Mathematics, 08.10.2019 05:30


Mathematics, 08.10.2019 05:30