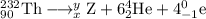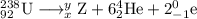Chemistry, 26.06.2019 19:30 chrissy5189
Write down the values of m (atomic number or atomic weight) and z (atomic number) for : (1) the element x formed from thorium-232 after six a and four b emissions? (2) the element y formed from uranium-238 after six a and two b emissions. (z=90 for thorium ; z=92 for uranium)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
Write down the values of m (atomic number or atomic weight) and z (atomic number) for : (1) the ele...
Questions

Health, 30.10.2020 14:00




History, 30.10.2020 14:00


Mathematics, 30.10.2020 14:00

Physics, 30.10.2020 14:00

Computers and Technology, 30.10.2020 14:00

History, 30.10.2020 14:00

Mathematics, 30.10.2020 14:00




Mathematics, 30.10.2020 14:00


Advanced Placement (AP), 30.10.2020 14:00


Mathematics, 30.10.2020 14:00