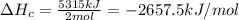Butane (c4 h10(g), hf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2 , hf = –393.5 kj/mol ) and water (h2 o, hf = –241.82 kj/mol) according to the equation below. what is the enthalpy of combustion (per mole) of c4h10 (g)? use . –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Butane (c4 h10(g), hf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2 , hf = –393...
Questions





Arts, 26.01.2021 03:20

Mathematics, 26.01.2021 03:20

Geography, 26.01.2021 03:20


English, 26.01.2021 03:20

Mathematics, 26.01.2021 03:20

English, 26.01.2021 03:20





Social Studies, 26.01.2021 03:20


Mathematics, 26.01.2021 03:20

Mathematics, 26.01.2021 03:20