
Chemistry, 26.06.2019 17:30 tynitenaire
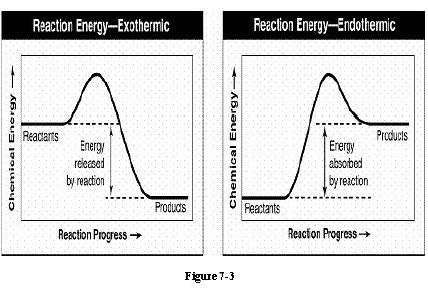
The bonds in the reactants of figure 7-3 contained 372 kj of chemical energy and the bonds in the products contained 350 kj of chemical energy. what is the amount of energy change during the reaction (show your work for full credit)? would this energy be absorbed or released? explain how you know.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which of the following is considered a benefit to using wind energy as a source of power
Answers: 1


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
The bonds in the reactants of figure 7-3 contained 372 kj of chemical energy and the bonds in the pr...
Questions

English, 22.10.2020 16:01

Arts, 22.10.2020 16:01

Mathematics, 22.10.2020 16:01








History, 22.10.2020 16:01



SAT, 22.10.2020 16:01






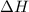 : This value is negative for exothermic reactions and positive for endothermic reactions.
: This value is negative for exothermic reactions and positive for endothermic reactions.

