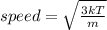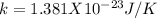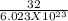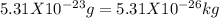Chemistry, 26.06.2019 17:00 phyllides4930
The average speed of oxygen molecules in air is about (1 point) 0 km/h 170 km/h 1700 km/h 17,000 km/h

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
The average speed of oxygen molecules in air is about (1 point) 0 km/h 170 km/h 1700 km/h 17,000 km...
Questions

Social Studies, 21.08.2019 15:30


Mathematics, 21.08.2019 15:30

French, 21.08.2019 15:30



Physics, 21.08.2019 15:30

Mathematics, 21.08.2019 15:30






Mathematics, 21.08.2019 15:30


Mathematics, 21.08.2019 15:30

Mathematics, 21.08.2019 15:30


Biology, 21.08.2019 15:30

History, 21.08.2019 15:30