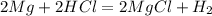Chemistry, 26.06.2019 17:00 catherinesquitieri
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the test tube, how would that mass compare to the mass of reactants left in the test tube after the reaction? explain your answer and how it corresponds to the law of conservation of mass.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the t...
Questions

Mathematics, 23.04.2021 05:50










English, 23.04.2021 06:00




Chemistry, 23.04.2021 06:00


Mathematics, 23.04.2021 06:00

Mathematics, 23.04.2021 06:00


Mathematics, 23.04.2021 06:00