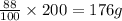1.you have a 2.0m nacl stock solution available. what is the volume you must dilute to make 500 ml of a 0.50m nacl solution? 2. how many grams of nano3 will precipitate if a saturated solution of nano3 in 200 g of water at 50°c is cooled to 20oc? assume the following solubility values for nano3: 114.0g/100g h2o at 50oc; 88.0g/100g h2o at 20oc 3. write equations to show how these substances ionize or dissociate in water. nh4cl cu(no3)2 hc2h3o2 hgcl2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
1.you have a 2.0m nacl stock solution available. what is the volume you must dilute to make 500 ml o...
Questions


Mathematics, 10.10.2019 06:20

Mathematics, 10.10.2019 06:20

Biology, 10.10.2019 06:20



History, 10.10.2019 06:20

Business, 10.10.2019 06:20





Mathematics, 10.10.2019 06:20

Mathematics, 10.10.2019 06:20

Mathematics, 10.10.2019 06:20

Mathematics, 10.10.2019 06:20


Mathematics, 10.10.2019 06:20


Mathematics, 10.10.2019 06:20

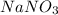 precipitate will be, 52 grams
precipitate will be, 52 grams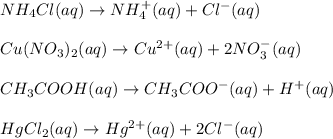
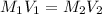
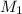 = concentration of NaCl stock solution = 2 M = 2 mole/L
= concentration of NaCl stock solution = 2 M = 2 mole/L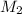 = concentration of NaCl solution = 0.50 M = 0.50 mole/L
= concentration of NaCl solution = 0.50 M = 0.50 mole/L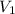 = volume of NaCl stock solution
= volume of NaCl stock solution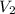 = volume of NaCl solution = 500 ml
= volume of NaCl solution = 500 ml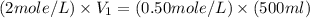
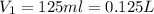 (1 L = 1000 ml)
(1 L = 1000 ml)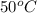 .
.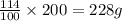
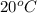 .
.