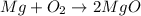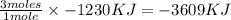Chemistry, 26.06.2019 15:00 trinity7265
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how much heat (in kj) is released when you completely react 3.000 moles of o2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how muc...
Questions


History, 04.03.2020 16:28



Social Studies, 04.03.2020 16:29




Biology, 04.03.2020 16:29



Mathematics, 04.03.2020 16:30



Chemistry, 04.03.2020 16:31


History, 04.03.2020 16:31


History, 04.03.2020 16:33

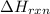 = -1230 KJ
= -1230 KJ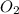 = 3 moles
= 3 moles