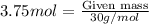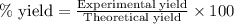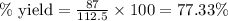Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
The first step in the ostwald process for producing nitric acid is 4nh3(g) + 5o2(g) -> 4no(g) +...
Questions


English, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40


Mathematics, 03.12.2020 18:40




English, 03.12.2020 18:40



Business, 03.12.2020 18:40







Mathematics, 03.12.2020 18:40

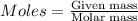
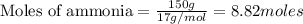
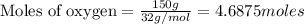
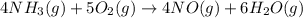
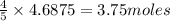 of ammonia
of ammonia