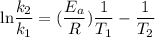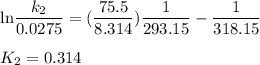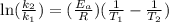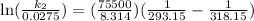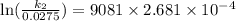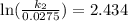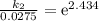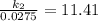Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1


Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2
You know the right answer?
Acertain first-order reaction has a rate constant of 2.75 10-2 s−1 at 20.°c. what is the value of k...
Questions


Mathematics, 10.12.2020 18:50





Social Studies, 10.12.2020 18:50



History, 10.12.2020 18:50


Health, 10.12.2020 18:50

Mathematics, 10.12.2020 18:50

Mathematics, 10.12.2020 18:50


Arts, 10.12.2020 18:50


Physics, 10.12.2020 18:50

Mathematics, 10.12.2020 18:50

Mathematics, 10.12.2020 18:50

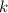 (rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.
(rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.