
Chemistry, 26.06.2019 11:30 tiffanydowell13
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are formed when 55.8 g or fe reacts completely with oxygen? 4fe(s)+3o 2(g) --> 2fe2o3(s) a. 0.25 mol b. 0. 50 mol c. o. 75 mol d. 1.00 mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are for...
Questions


Mathematics, 10.04.2020 11:00

English, 10.04.2020 11:00

Mathematics, 10.04.2020 11:01





Mathematics, 10.04.2020 11:01



Geography, 10.04.2020 11:01

History, 10.04.2020 11:01



Mathematics, 10.04.2020 11:01




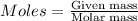
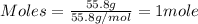
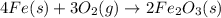
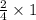 = 0.5 mole of Iron (III) oxide.
= 0.5 mole of Iron (III) oxide.

