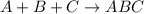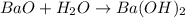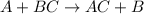Chemistry, 26.06.2019 10:30 LeahAshe123
What is the number of moles in 500 l of he gas at stp? 0.05 0.2 22 90 what is the percent of the composition of chromium bacro4? 4.87% 9.47% 20.5% 25.2% what is the empirical formula of a substance that is 53.5% c, 15.5% h, 31.1% n by weight c3hn2 c4h14n2 c2h7n ch4n7 the product of a combination reaction is ba(oh)2. if one of the reactants is h2o, what is the other reactant? ba2o bao bah bao2 which of the following is true about single replacement reactions they are restricted to metals they involve a single product two reactants produce two products any metal replaces any other metal. once all answered and finished i will post the whole exam. only if they are answered within 20 minutes. 11: 25am right now.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
What is the number of moles in 500 l of he gas at stp? 0.05 0.2 22 90 what is the percent of the co...
Questions

Business, 31.07.2019 06:30





History, 31.07.2019 06:30

History, 31.07.2019 06:30

Business, 31.07.2019 06:30


Biology, 31.07.2019 06:30


Spanish, 31.07.2019 06:30

Spanish, 31.07.2019 06:30

Spanish, 31.07.2019 06:30


Spanish, 31.07.2019 06:30




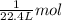
![]\frac{1}{22.4 L}\times 500L =22.32 mol\approx 22 mol](/tpl/images/0019/1602/e65bd.png)
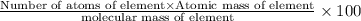
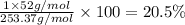
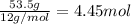
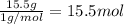
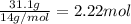
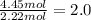
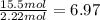
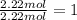
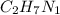 .
.