
Chemistry, 26.06.2019 01:30 BeenPaidGLO
What mass of fe can be produced by the reaction of 75.0 g co with excess fe2o3 according to the equation: fe2o3(s) + co(g) → fe(s) + co2(g)? a. 49.8 g fe b. 99.7 g fe c. 224 g fe d. 299 g fe

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
What mass of fe can be produced by the reaction of 75.0 g co with excess fe2o3 according to the equa...
Questions








History, 14.07.2019 15:00









Social Studies, 14.07.2019 15:00

Health, 14.07.2019 15:00


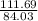 ×75.0 = 99.687g of Fe or 99.7g of Fe.
×75.0 = 99.687g of Fe or 99.7g of Fe.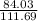 ×49.8 = 37.467g of CO should react. Thus the option is wrong.
×49.8 = 37.467g of CO should react. Thus the option is wrong.

