
What is the theoretical yield of copper produced by the reaction of: 0.5 g al reacted with 3.5 g cucl2 x h2o? (cucl2 x h2o is a dihydrate when calculating the molecular weight) hint.) first find the limiting reactant, then the molecular weights of copper by the aluminum and the (copper chloride times water).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3
You know the right answer?
What is the theoretical yield of copper produced by the reaction of: 0.5 g al reacted with 3.5 g cu...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








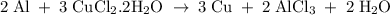
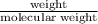
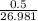
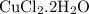 in reaction =
in reaction = 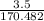
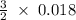
 170.482 grams of
170.482 grams of 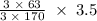 grams of Cu
grams of Cu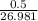 = 0.018 moles of Al.
= 0.018 moles of Al.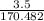 = 0.020 moles of CuCl₂.2H₂O.
= 0.020 moles of CuCl₂.2H₂O.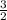 ×0.018 = 0.027.
×0.018 = 0.027.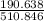 ×3.5 = 1.306g of Cu will produce.
×3.5 = 1.306g of Cu will produce.

