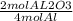Chemistry, 25.06.2019 21:30 madelyngv97
Given the following equation: 4al + 3o2 → 2al2o3. how many grams of aluminum oxide would form from reaction if 12.5 moles of aluminum burned?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Given the following equation: 4al + 3o2 → 2al2o3. how many grams of aluminum oxide would form from...
Questions

Mathematics, 26.02.2021 23:30

History, 26.02.2021 23:30




Mathematics, 26.02.2021 23:30


Mathematics, 26.02.2021 23:30

Chemistry, 26.02.2021 23:30



Mathematics, 26.02.2021 23:30

History, 26.02.2021 23:30

Spanish, 26.02.2021 23:30

Chemistry, 26.02.2021 23:30

History, 26.02.2021 23:30



English, 26.02.2021 23:30

Mathematics, 26.02.2021 23:30