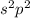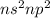Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 16:00
Challenge question: this question is worth 6 points. as you saw in problem 9 we can have species bound to a central metal ion. these species are called ligands. in the past we have assumed all the d orbitals in some species are degenerate; however, they often are not. sometimes the ligands bound to a central metal cation can split the d orbitals. that is, some of the d orbitals will be at a lower energy state than others. ligands that have the ability to cause this splitting are called strong field ligands, cnâ’ is an example of these. if this splitting in the d orbitals is great enough electrons will fill low lying orbitals, pairing with other electrons in a given orbital, before filling higher energy orbitals. in question 7 we had fe2+, furthermore we found that there were a certain number (non-zero) of unpaired electrons. consider now fe(cn)6 4â’: here we also have fe2+, but in this case all the electrons are paired, yielding a diamagnetic species. how can you explain this?
Answers: 2

You know the right answer?
Which of the following could be the subshell representation of an element of group iv? s2 s2pl s2p3...
Questions

History, 21.02.2020 08:20

Mathematics, 21.02.2020 08:21


Mathematics, 21.02.2020 08:21

History, 21.02.2020 08:21

History, 21.02.2020 08:21

Mathematics, 21.02.2020 08:22


Mathematics, 21.02.2020 08:22



Chemistry, 21.02.2020 08:23

Biology, 21.02.2020 08:24



History, 21.02.2020 08:24

History, 21.02.2020 08:25

Mathematics, 21.02.2020 08:25