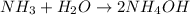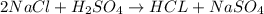Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
Which one of the following chemical equations is balanced? a. 2na + s → 2nas b. koh + h2so4 → khso...
Questions




Law, 27.10.2021 22:20

Social Studies, 27.10.2021 22:20

English, 27.10.2021 22:20


Mathematics, 27.10.2021 22:20

English, 27.10.2021 22:20



Mathematics, 27.10.2021 22:20

Chemistry, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20



Mathematics, 27.10.2021 22:20



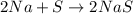
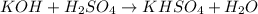
![[(39+16+1)+((2\times 1)+32+(4\times 16))]=154g/mol](/tpl/images/0016/2841/d0994.png)
![[(39+1+32+(4\times 16))+((2\times 1)+16)]=154g/mol](/tpl/images/0016/2841/a82d7.png)