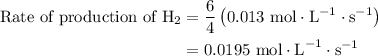Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
For the reaction: 4ph3(g) → p4(g) + 6h2(g) about 0.065 mol/s of ph3 is consumed in a 5.0 l flask. w...
Questions








Computers and Technology, 20.02.2020 01:51

History, 20.02.2020 01:51

Mathematics, 20.02.2020 01:52

Mathematics, 20.02.2020 01:52

Computers and Technology, 20.02.2020 01:52





Computers and Technology, 20.02.2020 01:52



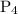 and
and 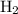 are
are 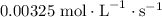 and
and 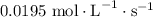 respectively.
respectively.

![{\text{Rate of reaction}} = - \dfrac{1}{{\text{a}}}\dfrac{{d\left[ {\text{A}} \right]}}{{dt}} = - \dfrac{1}{{\text{b}}}\dfrac{{d\left[ {\text{B}} \right]}}{{dt}} = \dfrac{1}{{\text{c}}}\dfrac{{d\left[ {\text{C}} \right]}}{{dt}} = \dfrac{1}{{\text{d}}}\dfrac{{d\left[ {\text{D}} \right]}}{{dt}}](/tpl/images/0015/9793/1d76f.png)
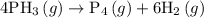
![{\text{Rate of reaction}}= - \dfrac{1}{4}\dfrac{{d\left[ {{\text{P}}{{\text{H}}_{\text{3}}}} \right]}}{{dt}} = \dfrac{{d\left[ {{{\text{P}}_{\text{4}}}} \right]}}{{dt}} = \dfrac{1}{6}\dfrac{{d\left[ {{{\text{H}}_2}} \right]}}{{dt}}](/tpl/images/0015/9793/a7105.png) …… (1)
…… (1)
![\dfrac{{d\left[ {{\text{P}}{{\text{H}}_{\text{3}}}} \right]}}{{dt}}](/tpl/images/0015/9793/9005a.png) is the rate of consumption of
is the rate of consumption of 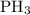 .
.
![\dfrac{{d\left[ {{{\text{P}}_{\text{4}}}} \right]}}{{dt}}](/tpl/images/0015/9793/a3838.png) is the rate of formation of
is the rate of formation of 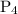 .
.
![\dfrac{{d\left[ {{{\text{H}}_2}} \right]}}{{dt}}](/tpl/images/0015/9793/f5e79.png) is the rate of formation of
is the rate of formation of 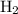 .
.
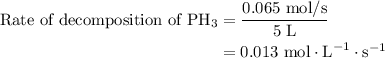
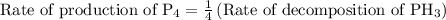 …… (2)
…… (2)
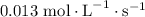 . Substitute this value in equation (2).
. Substitute this value in equation (2).
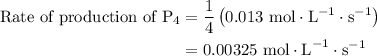
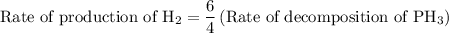 …… (3)
…… (3)