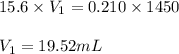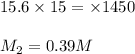Chemistry, 25.06.2019 14:00 ayowazzzgood
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to make 1450 ml of 0.210 m nh3? if you take a 15.0-ml portion of the stock solution and dilute it to a total volume of 0.600 l , what will be the concentration of the final solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to...
Questions




Geography, 27.03.2021 06:10


Mathematics, 27.03.2021 06:10

History, 27.03.2021 06:10

Mathematics, 27.03.2021 06:10

Mathematics, 27.03.2021 06:10



History, 27.03.2021 06:10

English, 27.03.2021 06:10


Biology, 27.03.2021 06:10


Mathematics, 27.03.2021 06:10

Mathematics, 27.03.2021 06:10

Mathematics, 27.03.2021 06:10

Mathematics, 27.03.2021 06:10

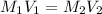
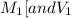 are the molarity and volume of one solution
are the molarity and volume of one solution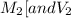 are the molarity and volume of another solution
are the molarity and volume of another solution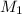 = Molarity of the stock solution = 15.6M
= Molarity of the stock solution = 15.6M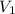 = Volume of the stock solution = ? mL
= Volume of the stock solution = ? mL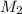 = Molarity of the diluted solution = 0.210M
= Molarity of the diluted solution = 0.210M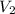 = Volume of the diluted solution = 1450 mL
= Volume of the diluted solution = 1450 mL