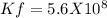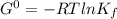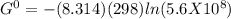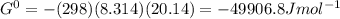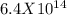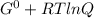Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which directio...
Questions


Computers and Technology, 30.03.2020 22:44




Mathematics, 30.03.2020 22:45





Computers and Technology, 30.03.2020 22:45




Computers and Technology, 30.03.2020 22:45