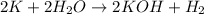When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2k(s) + 2h2o(l) → 2koh(aq) + h2(g). your lab partner says this is a redox reaction and a combustion reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2
You know the right answer?
When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide...
Questions


Mathematics, 11.06.2021 14:00


Mathematics, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00



World Languages, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00


Geography, 11.06.2021 14:00

Health, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00




English, 11.06.2021 14:00

Health, 11.06.2021 14:00