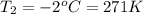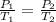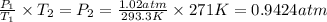Chemistry, 25.06.2019 11:00 MyChannelBruh6896
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle that has a pressure of 1.02 atm and a temperature of 20.3°c. it was placed in an ice chest whose internal temperature is -2.0°c. what will be the new pressure of the gas sample once the gas temperature in the jar equilibrates to that of the ice chest?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle...
Questions





Computers and Technology, 26.12.2019 23:31















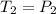
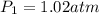 ,
, 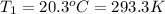
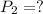 ,
,