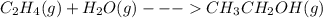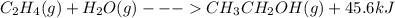When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of c2h4(g) that reacts. write a balanced thermochemical equation for the reaction with an energy term in kj as part of the equation. note that the answer box for the energy term is case sensitive. use the smallest integer coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. if a box is not needed, leave it blank. + + +?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

You know the right answer?
When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of...
Questions

Chemistry, 21.03.2020 10:31




Mathematics, 21.03.2020 10:31

Computers and Technology, 21.03.2020 10:31








Computers and Technology, 21.03.2020 10:32


History, 21.03.2020 10:32




Mathematics, 21.03.2020 10:32