
Chemistry, 25.06.2019 05:30 sanociahnoel
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal can be formed, and how many moles of the excess reactant will be left over when the reaction is complete? unbalanced equation: zn + agno3 → zn(no3)2 + ag be sure to show all of your work.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal ca...
Questions

Mathematics, 12.12.2020 16:20

Physics, 12.12.2020 16:20


Biology, 12.12.2020 16:20

History, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20



Arts, 12.12.2020 16:20

History, 12.12.2020 16:20

Engineering, 12.12.2020 16:20

Biology, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

Spanish, 12.12.2020 16:20


Computers and Technology, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Health, 12.12.2020 16:20

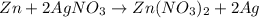
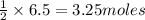 of zinc metal
of zinc metal of silver metal.
of silver metal.

