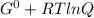Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
What is δg for the formation of solid uranium hexafluoride from uranium and fluorine at 25∘c when th...
Questions


Mathematics, 21.05.2021 23:00

Computers and Technology, 21.05.2021 23:00


Mathematics, 21.05.2021 23:00



Mathematics, 21.05.2021 23:00


History, 21.05.2021 23:00


Mathematics, 21.05.2021 23:00



Mathematics, 21.05.2021 23:00

Mathematics, 21.05.2021 23:00

Chemistry, 21.05.2021 23:00

History, 21.05.2021 23:00

Chemistry, 21.05.2021 23:00

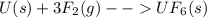
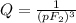
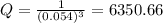
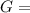 Δ
Δ